Asgari, P.; Hua, Y.; Bokka, A.; Thiamsiri, C.; Prasitwatcharakorn, W.; Karedath, A.; Chen, X.; Sardar, S.; Yum, K.; Leem, G.; Pierce, B. S.; Nam, K.; Gao, J.; Jeon, J.. Nature Catalysis, 2019, 2, 163-174.
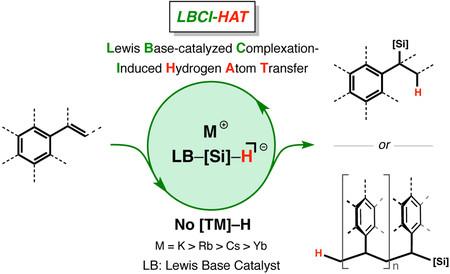
Because of the importance of hydrogen atom transfer (HAT) in biology and chemistry, there is increased interest in new strategies to perform HAT in a sustainable manner. Here, we describe a sustainable, net redox-neutral HAT process involving hydrosilanes and alkali metal Lewis base catalysts—eliminating the use of transition metal catalysts—and report an associated mechanism concerning Lewis base-catalysed, complexation-induced HAT. The catalytic Lewis base-catalysed, complexation-induced HAT is capable of accessing both branch-specific hydrosilylation and polymerization of vinylarenes in a highly selective fashion, depending on the Lewis base catalyst used. In this process, the Earth-abundant, alkali metal Lewis base catalyst plays a dual role. It first serves as a HAT initiator and subsequently functions as a silyl radical stabilizing group, which is critical to highly selective cross-radical coupling. An electron paramagnetic resonance study identified a potassiated paramagnetic species, and multistate density functional theory revealed a high HAT character, yet multiconfigurational nature in the transition state of the reaction.