Cote, J.M.; Ramirez-Mondragon, C.A.; Siegel, Z.S.; Czyzyk, D.J.; Gao, J.; Sham, Y.Y.; Mukerji, I.; Taylor, E.A. Biochemistry, 2017, 56 (6), 886-895.
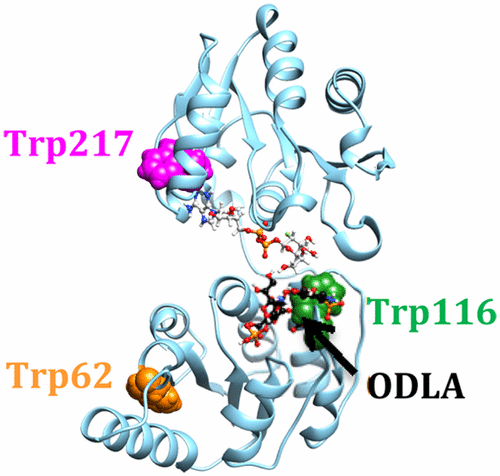
Heptosyltransferase I (HepI) catalyzes the addition of l-glycero-β-d-manno-heptose to Kdo2-Lipid A, as part of the biosynthesis of the core region of lipopolysaccharide (LPS). Gram-negative bacteria with gene knockouts of HepI have reduced virulence and enhanced susceptibility to hydrophobic antibiotics, making the design of inhibitors of HepI of interest. Because HepI protein dynamics are partially rate-limiting, disruption of protein dynamics might provide a new strategy for inhibiting HepI. Discerning the global mechanism of HepI is anticipated to aid development of inhibitors of LPS biosynthesis. Herein, dynamic protein rearrangements involved in the HepI catalytic cycle were probed by combining mutagenesis with intrinsic tryptophan fluorescence and circular dichroism analyses. Using wild-type and mutant forms of HepI, multiple dynamic regions were identified via changes in Trp fluorescence. Interestingly, Trp residues (Trp199 and Trp217) in the C-terminal domain (which binds ADP-heptose) are in a more hydrophobic environment upon binding of ODLA to the N-terminal domain. These residues are adjacent to the ADP-heptose binding site (with Trp217 in van der Waals contact with the adenine ring of ADP-heptose), suggesting that the two binding sites interact to report on the occupancy state of the enzyme. ODLA binding was also accompanied by a significant stabilization of HepI (heating to 95 °C fails to denature the protein when it is in the presence of ODLA). These results suggest that conformational rearrangements, from an induced fit model of substrate binding to HepI, are important for catalysis, and the disruption of these conformational dynamics may serve as a novel mechanism for inhibiting this and other glycosyltransferase enzymes.